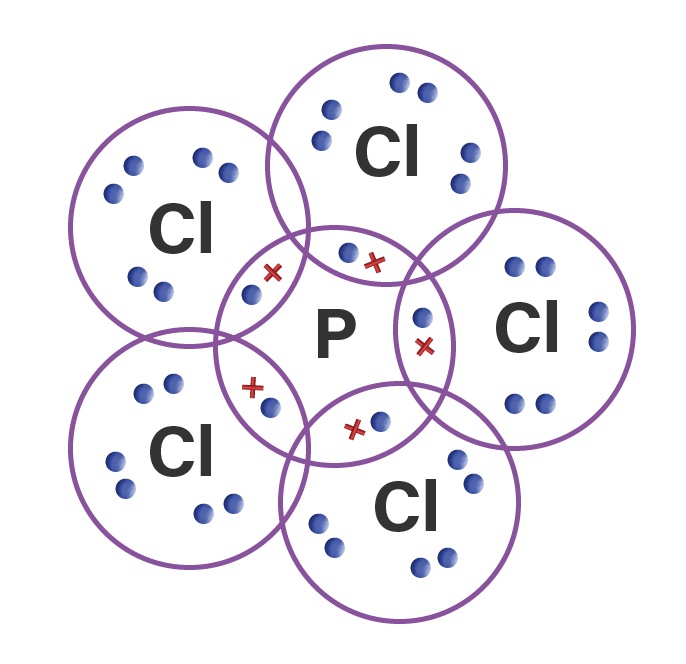
Gambarkan rumus Lewis dari molekul PCl5
PCl. 5. (Phosphorus pentachloride) Lewis Structure. Phosphorus pentachloride (PCl 5) contains five chlorine atoms and one phosphorus atom. In PCl 5 lewis structure, each chlorine atom is joint with center phosphorus atom through a single bond (sigma bond). You can see there is no lone pairs on phosphorus atom in PCl 5 as PCl 3.
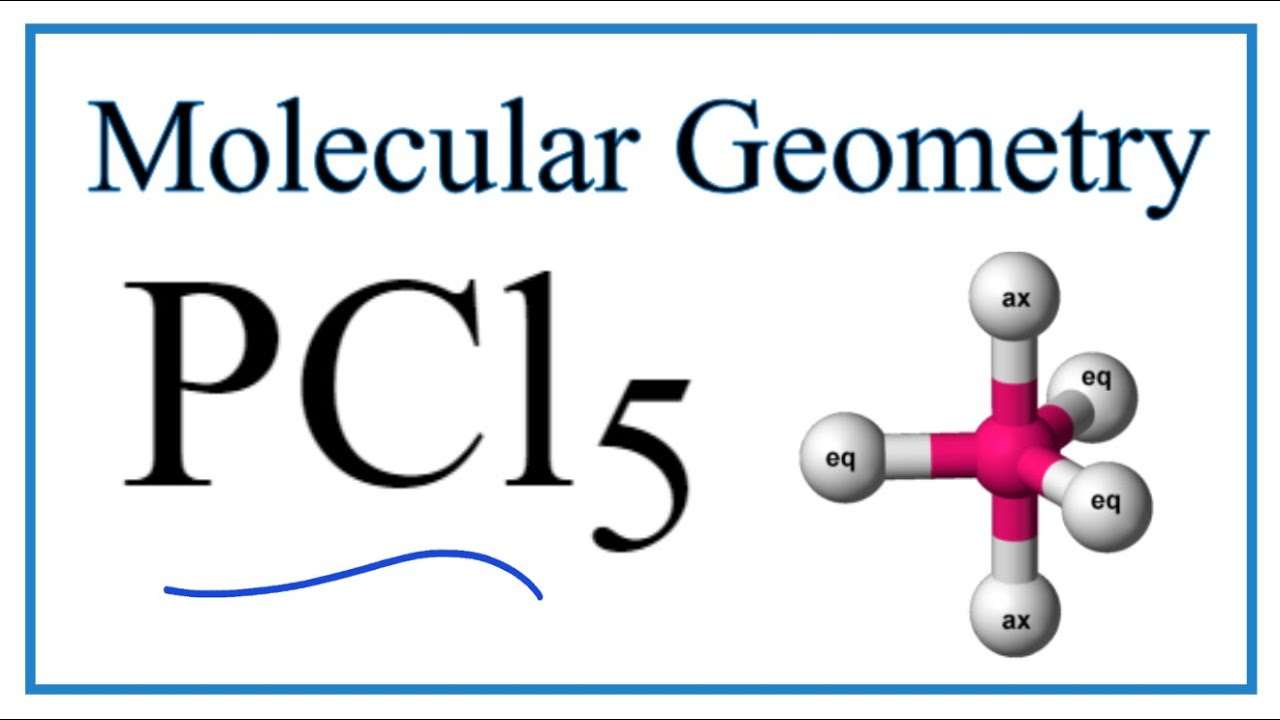
PCl5 (Phosphorus pentachloride) Molecular Geometry, Bond Angles YouTube
Steps for drawing PCl5 lewis structure. Let's draw the lewis dots for PCl 5 by following the below steps one by one: Find how many number of valence electrons are available for bonding in PCL 5. P (Z = 15) = [Ne] 3s²3p³ ie. 5 valence electrons are there for Phosphorous. Cl (Z = 17) = [Ne] 3s²3p⁵ ie. 7 electrons for each chlorine atom.

PCl5 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram Techiescientist
MO diagram depicts chemical and physical traits of a molecule like bond length, bond energy, bond angle, shape, etc. Following are the steps to design the MO diagram of PCl5 : Step 1: Identify the valence electrons of each atom. In PCl5, it is 5 for P and 7 for every 5 atoms of Cl. Step 2: Check if the molecule is heteronuclear or homonuclear.

Draw lewis structures of PCl5 Brainly.in
Another (easier) method to determine the Lewis structure of PCl 5: Alternatively a dot method can be used to draw the Lewis structure. Calculate the total valence electrons in the molecule. P: 1×5 = 5. Cl: 5×7 = 35. Total = 40 valence electrons. Now, treat the atoms and electrons like puzzle pieces.

Pembahasan soal Struktur Lewis Bentuk molekul PCl5 YouTube
Steps. Use these steps to correctly draw the PCl 5 Lewis structure: #1 First draw a rough sketch. #2 Mark lone pairs on the atoms. #3 Calculate and mark formal charges on the atoms, if required. Let's discuss each step in more detail.

25. Lewis Dot Structure of PCl5 How to Draw Lewis Structures Class 11 Chemistry Chemical
I quickly take you through how to draw the Lewis Structure of PCl5, phosphorous pentachloride. I also go over formal charge, hybridization, shape and bond an.
William Of Wales pcl5 lewis structure
Dalam molekul NH 3 terdapat sepasang elektron yang tidak digunakan (elektron bebas) sehingga disebut Pasangan Elektron Bebas (PEB). Tiga pasang elektron yang digunakan bersama oleh atom N dan atom H disebut Pasangan Elektron Ikatan (PEI). 2. Struktur Lewis Molekul H 2 O. Atom 8 O memiliki konfigurasi elektron 8 O:2, 6.

Lewis Structure Pcl5
Check me out: http://www.chemistnate.com
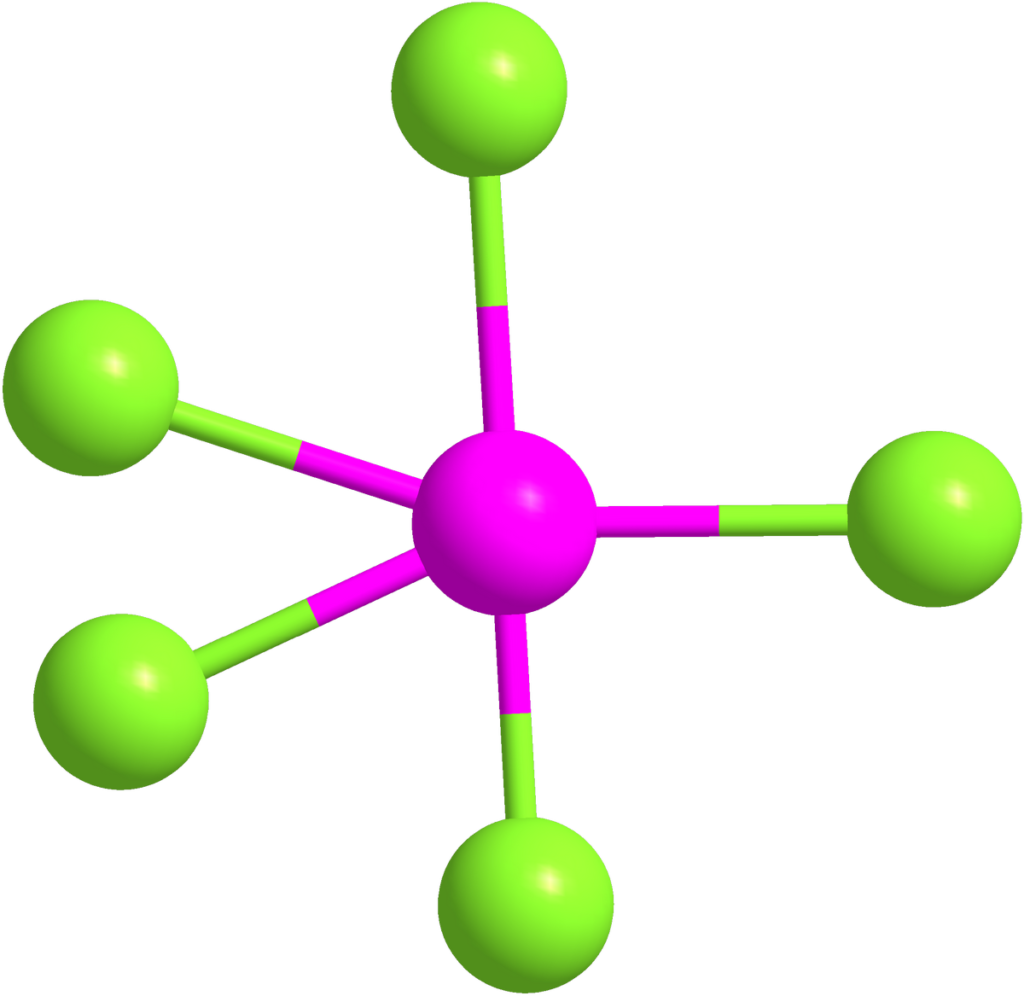
Bentuk Molekul PCl5 MateriKimia
Lewis Structures. Page ID. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the "best" electron.

Lewis Structure of PCl5 [with free study guide and video]
There are a total of 40 valence electrons in the PCl5 Lewis structure. Remember when you draw the Lewis structure for PCl5 that Phosphorous (P) is in Period 3 on the Periodic table. This means that it can hold more than 8 valence electrons. For the Lewis structure for PCl5 you should take formal charges into account to find the best Lewis.

what is the name for the compound pcl5?
Drawing the Lewis Structure for PCl 5. PCl 5 is similar to PBr 5 and PF 5. If you can do those Lewis structures PCl 5 will be easy. In the PCl 5 Lewis structure Phosphorus (P) is the least electronegative so it goes in the center. In the Lewis structure for PCl 5 there are a total of 40 valence electrons. Five pairs will be used in the chemical.

Pcl5 Lewis Structure / Figure 5.5 lewis symbols for the elements of the first three periods
A step-by-step explanation of how to draw the PCl5 Lewis Dot Structure (Phosphorus pentachloride).For the PCl5 structure use the periodic table to find the t.
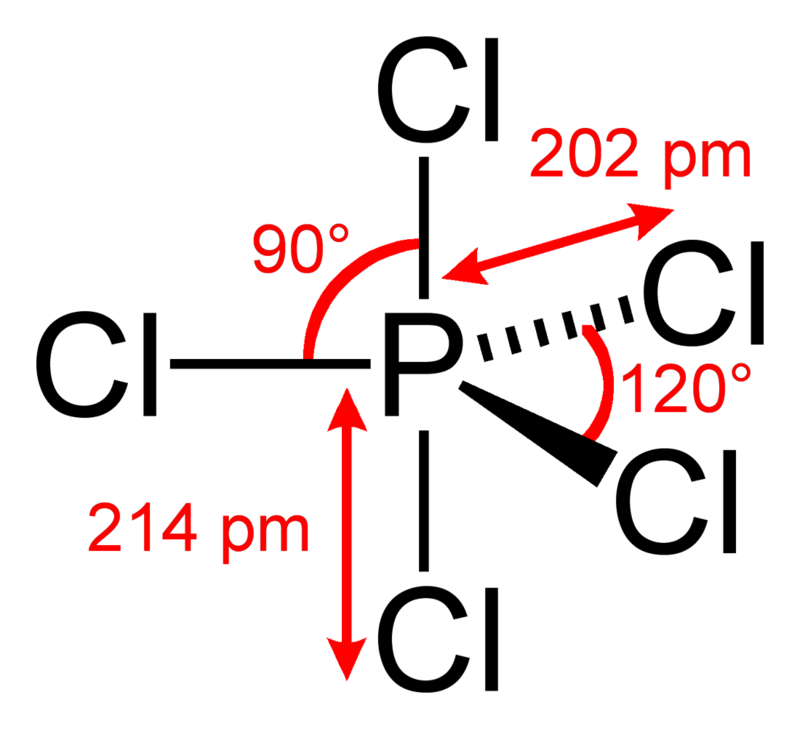
What is the structure of PCl_5? + Example
In the PCl 5 Lewis dot structure, a total of 15 lone pairs and 5 bond pairs are present. The electron geometry of PCl 5 is also Trigonal bipyramidal. The hybridization of phosphorous in PCl 5 is sp 3 d. Since its steric number is 5. In PCl 5, axial atoms (2 P-Cl bonds) make a 90º angle with the plane, and equatorial atoms (3 P-Cl bonds) make a.

PCl5 lewis structure, molecular geometry, hybridization, bond angle
PCl5 Lewis Structure, Molecular Structure, Hybridization, Bond Angle, and Shape. The chemical formula PCl 5 represents the chemical compound Phosphorus Pentachloride. It is a greenish-yellow crystalline solid with an irritating odor. It is mainly used as a chlorinating reagent.
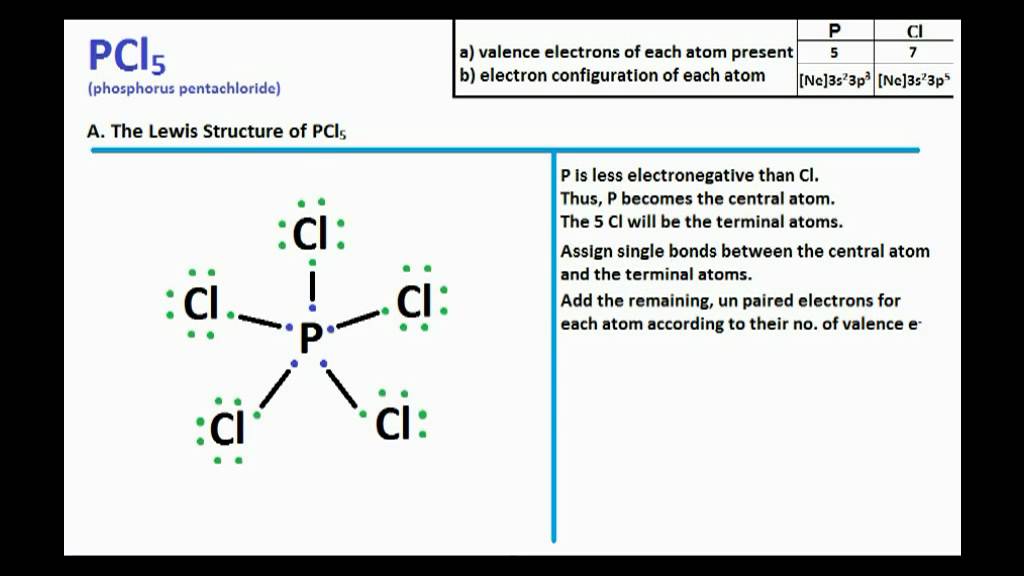
PCl5 Lewis Structure and Molecular Geometry YouTube
Steps of drawing PCl5 lewis structure Step 1: Find the total valence electrons in PCl5 molecule. In order to find the total valence electrons in PCl5 (phosphorus pentachloride) molecule, first of all you should know the valence electrons present in phosphorus atom as well as chlorine atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.)

QUIMICA Estructura de Lewis PCl5 Carga Formal e hibridación sp3d Expansión Octeto AULAEXPRESS
in this video you will learn how to draw Lewis structure (Lewis formula) for PCl5 (Phosphorous Pentachloride).Lewis structure Playlist: https://www.youtube.c.