
Buatlah Struktur Lewis Nh3
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

Struktur Lewis senyawa NH3BF3 adalah sebagai berikut H F...
Beranda. Perhatikan rumus struktur Lewis dari NH 3 BF 3 ber. Iklan. Pertanyaan. Perhatikan rumus struktur Lewis dari NH 3 BF 3 berikut! Pasangan elektron yang membentuk ikatan kovalen koordinasi ditunjukkan oleh nomor. (Nomor atom H = 1; N = 7; B = 5; F = 9) 1. 2.

BF3 Lewis Structure How to Draw the Lewis Structure for BF3 YouTube
Dalam molekul NH 3 terdapat sepasang elektron yang tidak digunakan (elektron bebas) sehingga disebut Pasangan Elektron Bebas (PEB). Tiga pasang elektron yang digunakan bersama oleh atom N dan atom H disebut Pasangan Elektron Ikatan (PEI). 2. Struktur Lewis Molekul H 2 O. Atom 8 O memiliki konfigurasi elektron 8 O:2, 6.

Gambarkan Struktur Lewis Nh3
Step #1: Calculate the total number of valence electrons. Here, the given molecule is NH3 (ammonia). In order to draw the lewis structure of NH3, first of all you have to find the total number of valence electrons present in the NH3 molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom).

NH3 And BF3 form an adduct readily because they form
Pertanyaan. Perhatikan rumus struktur Lewis dari NH3BF3 berikut! Pasangan elektron yang ikatan kovalen koordinasi oleh nomor membentuk ditunjukkan (Nomor atom H = 1 ; N = 7; B = 5; F = 9 ) (A) 1 (B) 2 (C) 3 (D) 4 (E) 5

BF3 Lewis Structure (Boron Trifluoride) YouTube
A step-by-step explanation of how to draw the NH3 Lewis Dot Structure (Ammonia).For the NH3 structure use the periodic table to find the total number of vale.

Struktur Lewis Senyawa Nh3Bf3 Sebagai Berikut Extra
Consider the following acid-base reaction:NH3 + BF3 ⇌ H3N+—BF3−1. For all the reactants and products, draw Lewis structures.2. Identify the nucleophile (base) and electrophile (acid) in the reaction.3. Draw curved arrows to show the flow of electrons.4. Determine if the reaction can be termed a Brønsted-Lowry acid-base reaction.

NH3 BF3
Pertanyaan. Perhatikan rumus struktur Lewis senyawa NH 4 Cl berikut! Ikatan kovalen koordinasi pada gambar tersebut ditunjukkan nomor. (Nomor atom N = 7; H = 1; Cl = 17) 1. 2. 3.
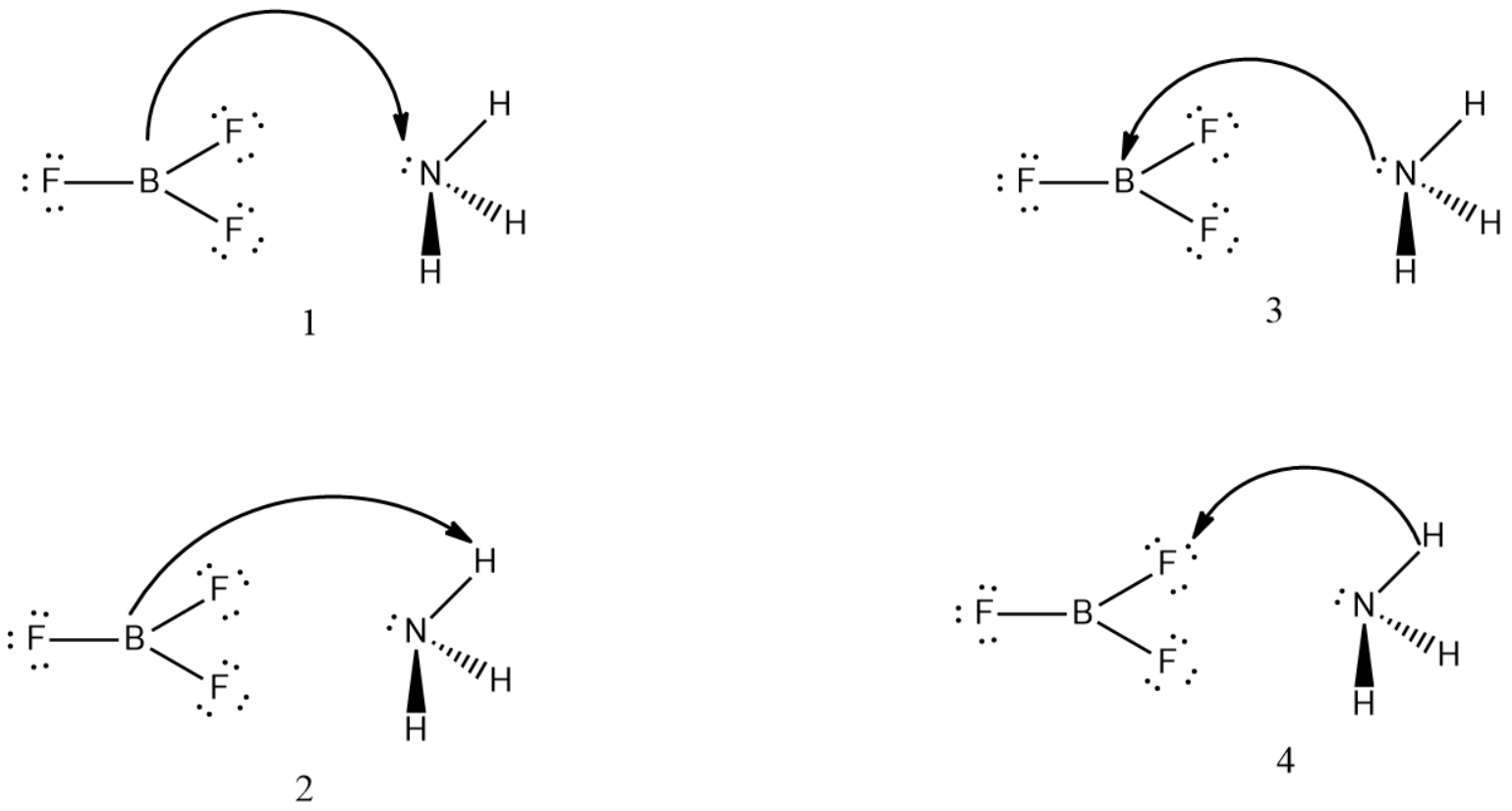
{ BF }_{ 3 } and { NH }_{ 3 } undergo Lewis acidbase reaction forming an adduct. Which
Boron Trifluoride (BF3) is an inorganic compound as it lacks a carbon atom or C-H bond in the molecule. Manufactured from the reaction of boron oxides and hydrogen fluoride, the chemical compound BF3 has a pungent smell and is colorless in nature. The compound behaves differently in different states of matter.

Gambarkan Struktur Lewis Senyawa Nh3 Ilmusosial & Pendidikan
Soal No. 1 Apa yang dimaksud dengan struktur Lewis? Pembahasan: Struktur lewis adalah penggambaran elektron valensi suatu atom dengan notasi (penulisan lambang atom dikelilingi oleh titik di sekitarnya). Soal No. 2 Gambarkan konfigurasi elektron dan struktur Lewis unsur-unsur di bawah ini! 11Na 6C 8O 17Cl 12Mg Pembahasan: 1. Konfigurasi elektron 11Na = 2, 8, 1
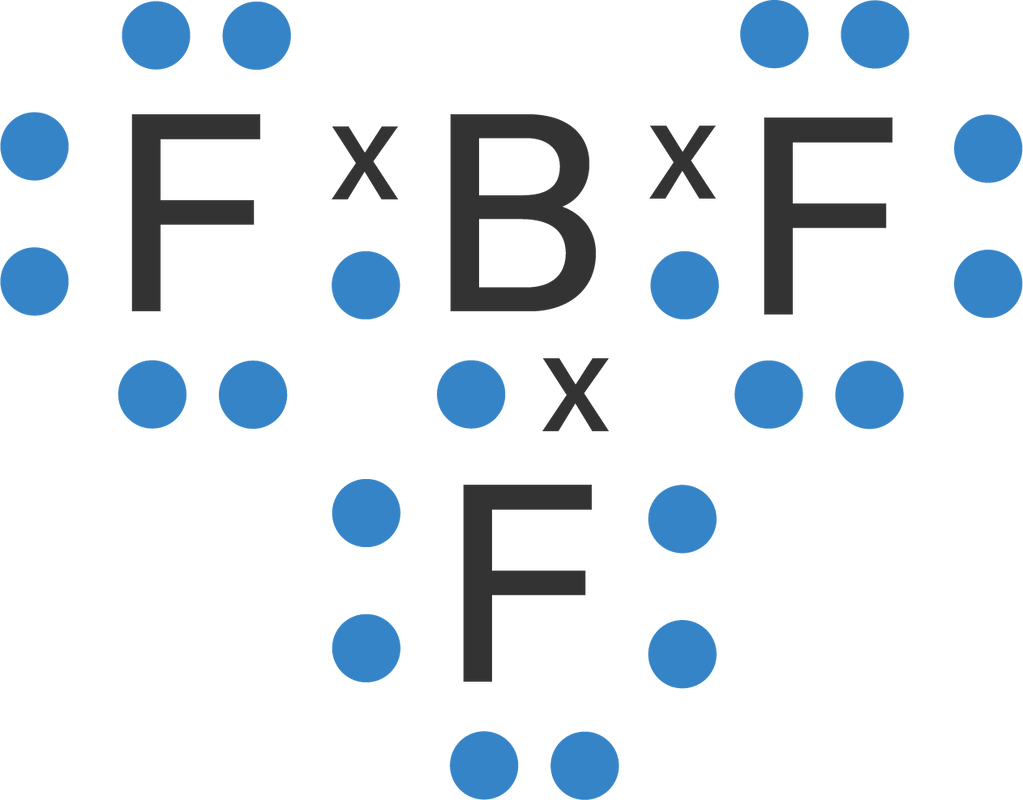
Tuliskan struktur Lewis dan struktur molekul dari
The Lewis electron structure for the NH 4+ ion is as follows: The nitrogen atom shares four bonding pairs of electrons, and a neutral nitrogen atom has five valence electrons. Using Equation 4.4.1, the formal charge on the nitrogen atom is therefore. formalcharge(N) = 5 −(0 + 8 2) = 0.
Molekul NH3BF3 terbentuk ketika amonia direaksikan...
BF3 Hybridization. Hybridization stands for mixing atomic orbitals into new hybrid orbitals. They are accommodating to explain molecular geometry and nuclear bonding properties. There are several types of hybridization like SP3, SP2, SP. BF3 is SP2 hybridization. For this molecule, It is SP2 because one π (pi) bond is required for the double.

Ácidos y bases. Teoría de Lewis Física Química
Section 7: Extensions of the Lewis Structure Model. Page ID. With these thoughts in mind, we turn to a set of molecules which challenge the limits of the Lewis model in describing molecular structures. First, we note that there are a variety of molecules for which atoms clearly must bond in such a way as to have more than eight valence electrons.

NH3 And BF3 form an adduct readily because they form
Ikut Bimbel online CoLearn mulai 95.000/bulan.IG CoLearn: @colearn.id https://bit.ly/Instagram-CoLearnSekarang, yuk latihan soal ini!Struktur Lewis senyawa N.

Lewis Dot Structure For Bf3
Explanation: The structure of H3N-BF3 is. The B and N atoms each have four single bonds, so their hybridizations are sp3 with bond angles of 109.5°. Answer link. Every bond angle is approximately 109.5°. > The structure of "H"_3"N-BF"_3 is The "B" and "N" atoms each have four single bonds, so their hybridizations are "sp"^3 with bond angles.

Estructura de Lewis NH3, Amoniaco » Quimica Online
Steps of drawing lewis structure of BF 3. There are general guidelines to draw a lewis structure step by step and they are mentioned below. In this lesson, we use those rules to draw the BF 3 lewis structure and they are explained in detail in next sections of this tutorial. If you are are beginner to lewis structure drawing, follow these sections slowly and properly to understand.