
piramida triunghiulara regulata Mate Pedia
The difference between trigonal planar and trigonal pyramidal can be seen in the structure of a molecule. Trigonal planar does not have a lone pair of electrons while trigonal pyramidal has a lone pair in the central atom. Also, all the atoms of a planar molecule lie in the same plane unlike the atoms of pyramidal.
Jelaskan arti dari istilah berikut! f. Piramida...
Conclusion. Trigonal planar and Trigonal pyramidal are two molecular geometries that are determined by the arrangement of atoms or groups around a central atom. Trigonal planar geometry has three atoms or groups arranged in a flat, triangular shape, while trigonal pyramidal geometry has three atoms or groups arranged in a pyramid shape.

piramida triunghiulara regulata Mate Pedia
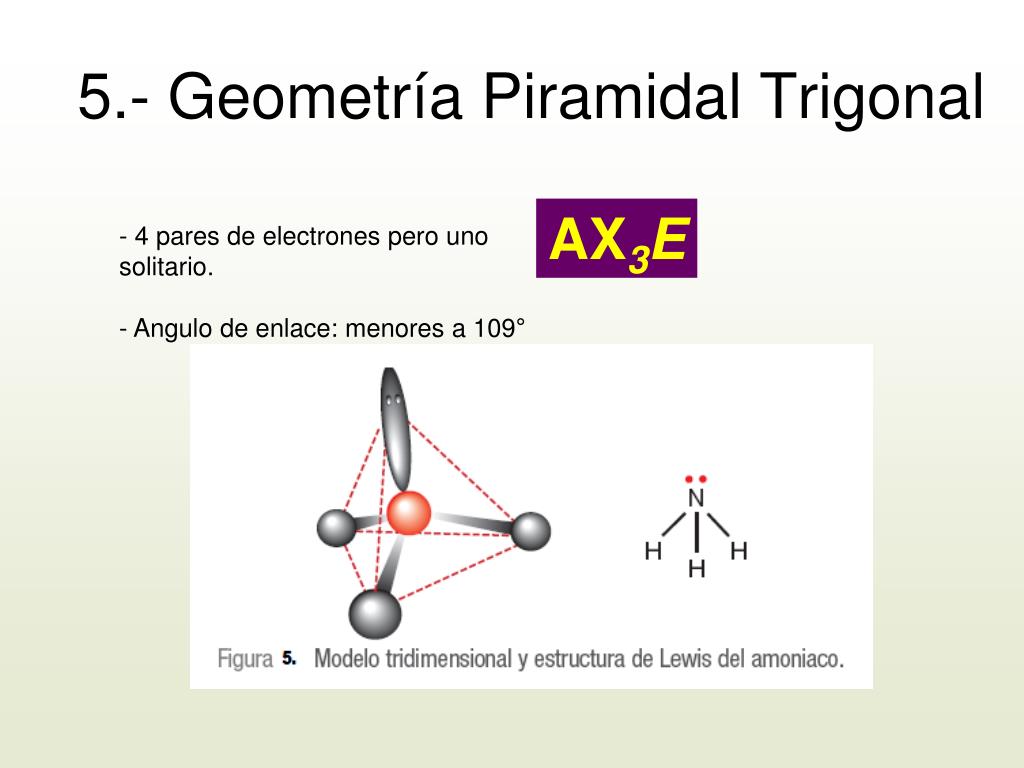
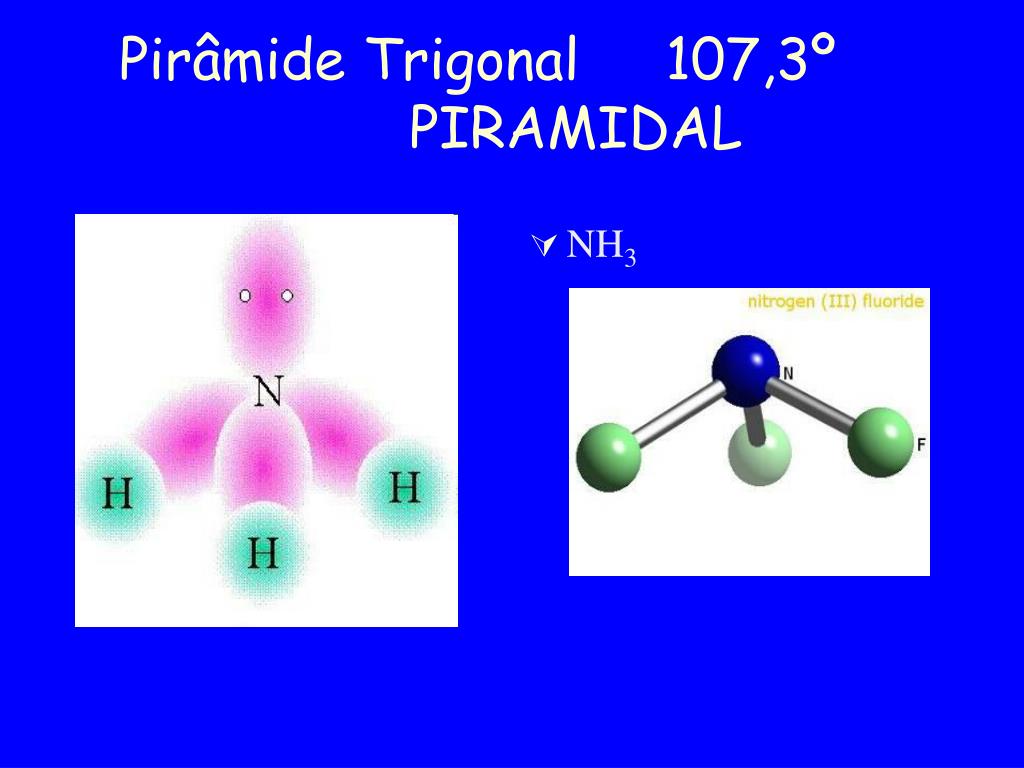
October 24, 2023. Trigonal pyramidal geometry is a shape found in compounds with a central atom that has four electron domains. It has three bonding pairs and one lone pair of electrons. The bond angles in this shape are about 107 degrees. Knowing about trigonal pyramidal geometry is important because it affects the properties of compounds.

Pyramid (geometry) Wikipedia
Stereoisomers. Since there are two types of atoms on a Trigonal Bipyramidal structure, axial and equatorial, there are different Stereoisomers that could arise depending on the substituents attached. For example, if there is 4 X atoms and 1 Y atom attached to the central atom, Y could either be in an equatorial position or in an axial position.
Trigonal Pyramidal Lewis Dot Structure
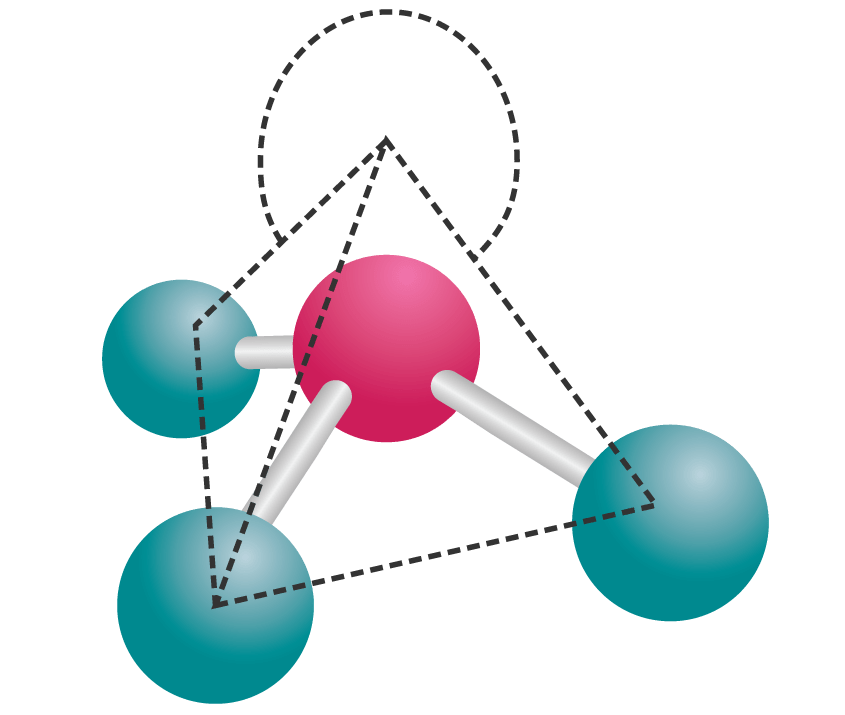
The trigonal pyramid is a molecular geometry that resembles a tetrahedron that has one atom at the apex and three atoms at the trigonal base corners. The molecule belongs to point group C3v because all three atoms present at the corners are equal. A few ions and molecules having trigonal pyramidal geometry are given as the xenon trioxide (XeO3.

Pirámide triángulo ostrosłup prawidłowy borde plano, pirámide, ángulo, cara, simetría png PNGWing
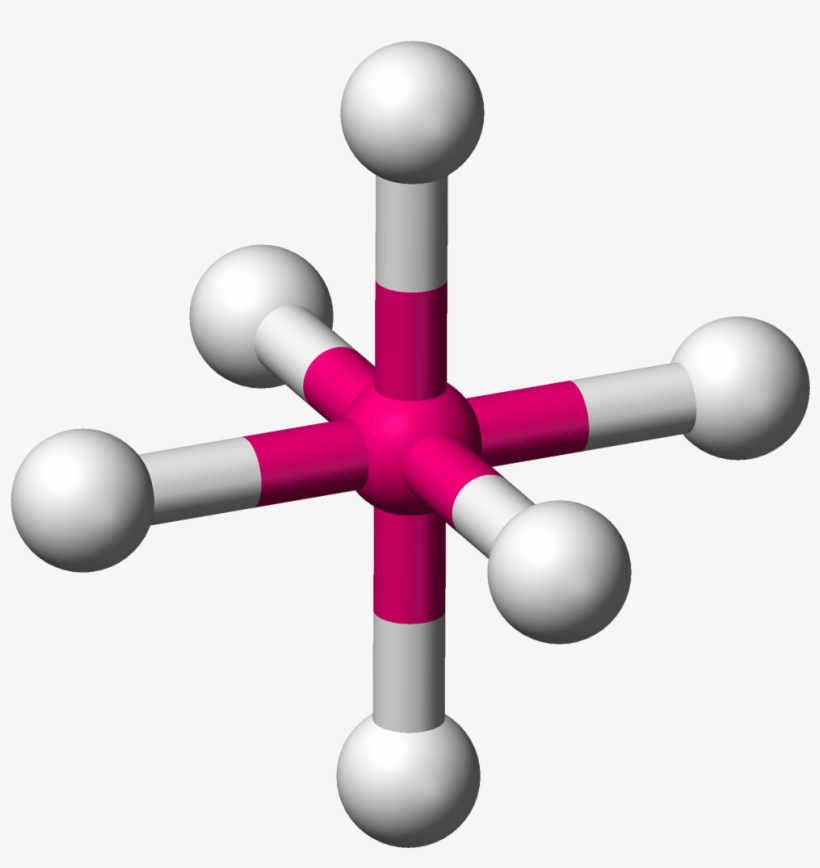
Trigonal bipyramidal molecular geometry. In chemistry, a trigonal bipyramid formation is a molecular geometry with one atom at the center and 5 more atoms at the corners of a triangular bipyramid. [1] This is one geometry for which the bond angles surrounding the central atom are not identical (see also pentagonal bipyramid ), because there is.

Molecular Geometry at Kaplan University (MO) StudyBlue
Orbit navigation Move camera: 1-finger drag or Left Mouse Button Pan: 2-finger drag or Right Mouse Button or SHIFT+ Left Mouse Button Zoom on object: Double-tap or Double-click on object Zoom out: Double-tap or Double-click on background Zoom: Pinch in/out or Mousewheel or CTRL + Left Mouse Button
How Many Bonds Are In A Trigonal Pyramidal? Mastery Wiki
A trigonal bipyramidal molecule is a bit more complicated, mostly because it has more atoms and bonds to take into account. As the name implies, a trigonal bipyramidal shape looks like two three.
terceroespañol Pirámides
Pharmaceutical and Medicine Manufacturing Newspaper, Periodical, Book, and Directory Publishers Media Streaming Distribution Services, Social Networks, and Other Media Networks and Content Providers Scientific Research and Development Services Business Support Services Soap, Cleaning Compound, and Toilet Preparation Manufacturing Miscellaneous Nondurable Goods Merchant Wholesalers Drugs and.
Aria Laterala Piramida Triunghiulara Regulata 8
In this video we'll look at the Trigonal Pyramidal Molecular Geometry and Bond Angles. We'll use the example of NH3 to understand the molecular shape. To.

Nh3 Geometria Piramidal Trigonal Trigonal Pyramid
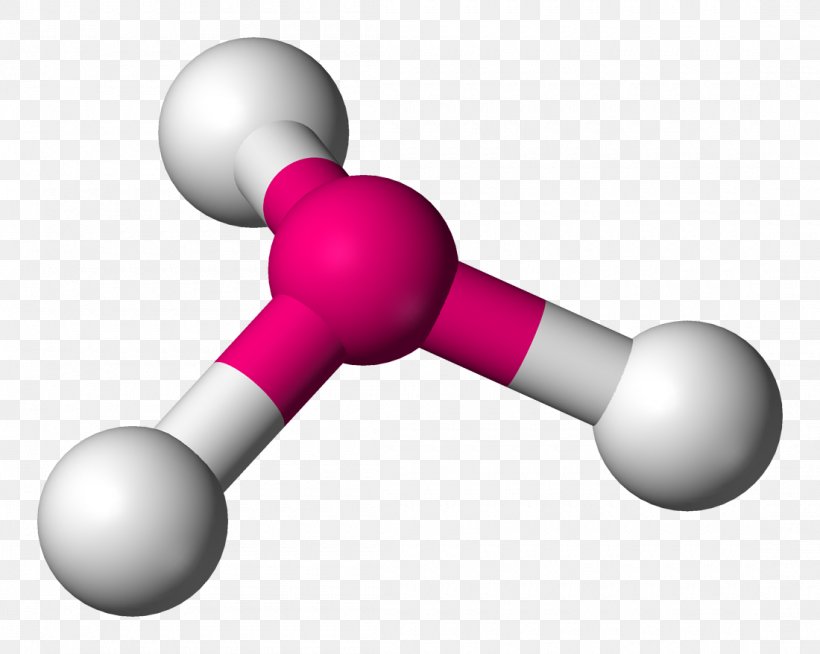
Trigonal pyramidal is a molecular shape that results when there are three bonds and one lone pair on the central atom in the molecule. Molecules with an tetrahedral electron pair geometries have sp3 hybridization at the central atom. Ammonia (NH 3) is a trigonal pyramidal molecule. → Download high quality image.
Pirâmide Características e fórmulas básicas VouPassar
An example of an ideal molecule with trigonal planar is Boron trifluoride. Examples of inorganic anions that show trigonal planar are carbonates and sulfates. Other complex compounds that normally surround central atoms are three NH2 groups and tend to be bind on the central atom. Differences Between Trigonal Planar and Trigonal Pyramidal

Piramida triunghiulara, tetraedrul descriere si reprezentare Mate Pedia
Trigonal pyramidal is a geometry of some molecules like ammonia and phosphine. Let us an example of ammonia to understand the trigonal pyramidal molecular geometry. Ammonia is an inorganic compound with three hydrogen atoms surrounding one nitrogen atom. All three hydrogen atoms shared their valence electrons with the nitrogen atom forming three strong covalent bonds. […]

Elongated Trigonal Pyramid Triangle Clipart Large Size Png Image PikPng
Other articles where trigonal pyramidal arrangement is discussed: ammonia: Physical properties of ammonia:.The ammonia molecule has a trigonal pyramidal shape with the three hydrogen atoms and an unshared pair of electrons attached to the nitrogen atom. It is a polar molecule and is highly associated because of strong intermolecular hydrogen bonding. The dielectric constant of ammonia (22.
PPT Enlace Químico PowerPoint Presentation, free download ID5777686
Trigonal pyramidal molecular geometry. In chemistry, a trigonal pyramid is a molecular geometry with one atom at the apex and three atoms at the corners of a trigonal base, resembling a tetrahedron (not to be confused with the tetrahedral geometry ). When all three atoms at the corners are identical, the molecule belongs to point group C3v.
PPT Geometria Molecular e Interações Químicas Moleculares PowerPoint Presentation ID3560795
Three orbitals are arranged around the equator of the molecule with bond angles of 120 o. Two orbitals are arranged along the vertical axis at 90 o from the equatorial orbitals. The shape of the orbitals is trigonal bipyramidal. Since there is an atom at the end of each orbital, the shape of the molecule is also trigonal bipyramidal.