
[MCQ] Assertion (A) HCl converts pepsinogen into active enzyme pepsin
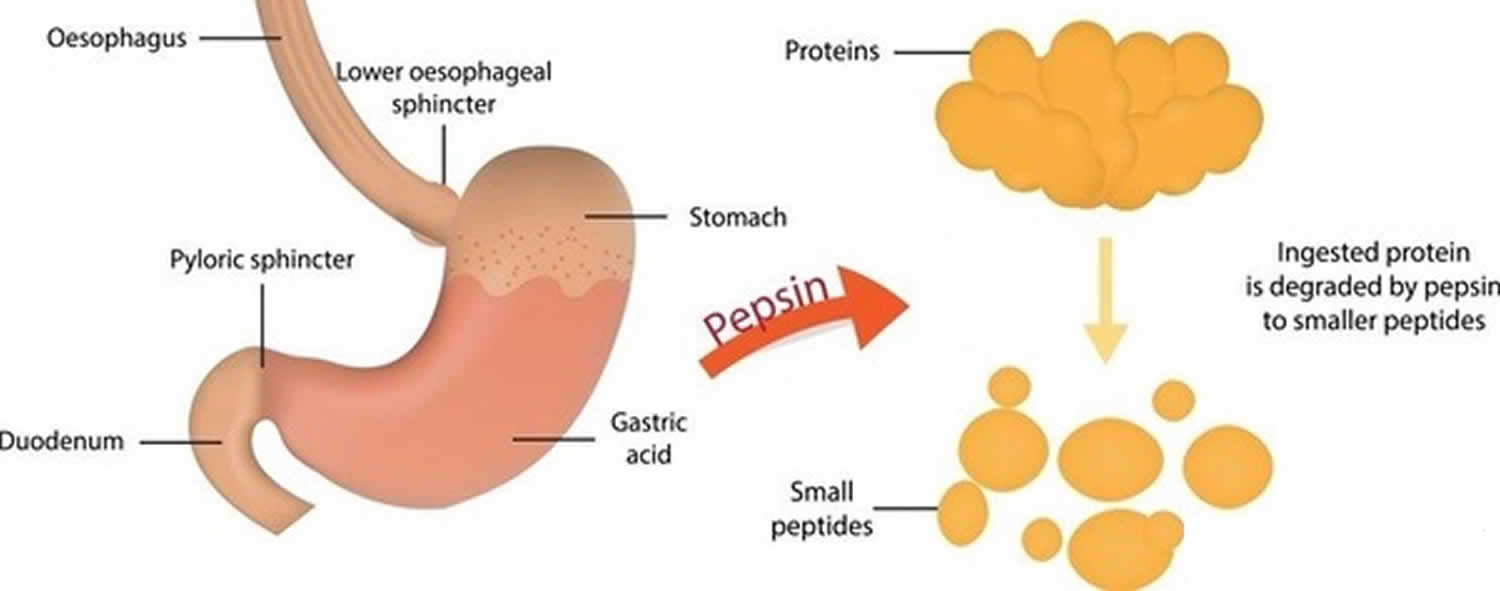
Saat makanan masuk ke dalam mulut, organ lambung akan segera memproduksi cairan yang terdiri dari air, lendir, enzim pencernaan, elektrolit, serta asam lambung. Namun, asam lambung ini yang berfungsi untuk mengubah pepsinogen menjadi enzim pepsin. Pepsinogen sendiri merupakan zat yang dibuat oleh sel-sel di lapisan dinding lambung.

What is Pepsin?
Pepsinogen has an additional 44 amino acids on its N-terminus. During the transformation of pepsinogen into pepsin, these 44 amino acids are released. While pepsin has fewer basic amino acid residues, it has 44 acidic residues. This is the reason why it remains stable at extremely low pH. To prevent self-digestion, pepsins need to be stored at.

Pepsin enzyme function, source of production and where is pepsin found
sebagai sumber enzim pepsin, misalnya dari lambung ikan tuna.. Methods 2, that also considers a pepsinogen concentration step by coagulation, and Method 3, that also considers a lyophilisation.
Pepsin Enzyme Structure, Function, and Important Facts Science Struck
ABSTRACT Studies on gastric digestion during 1820-1840 led to the discovery of pepsin as the agent which, in the presence of stomach acid, causes the dissolution of nutrients such as meat or coagulated egg white. Soon afterward it was shown that these protein nutrients were cleaved by pepsin to diffusible products named peptones. Efforts to isolate and purify pepsin were spurred by its.

RCSB PDB 1PSO The crystal structure of human pepsin and its complex with pepstatin
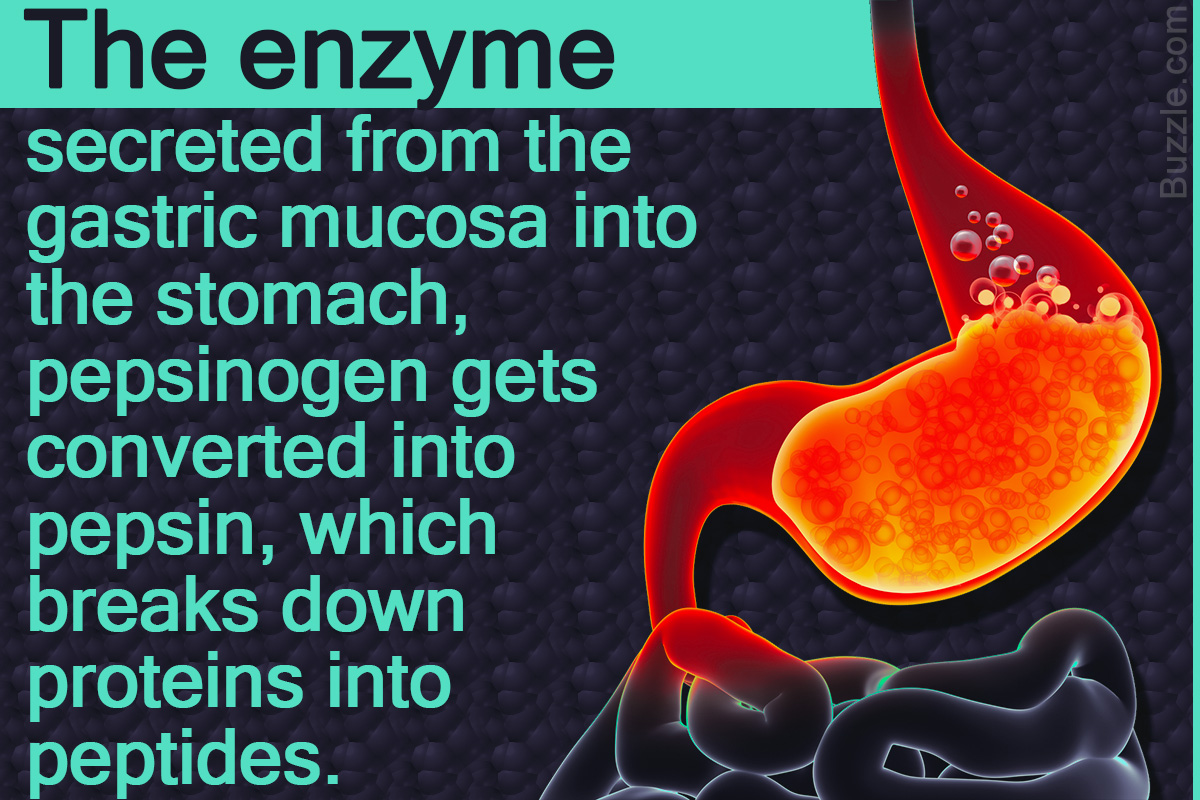
Pepsin adalah enzim yang memecah protein menjadi peptida yang lebih kecil (pepsin merupakan salah satu protease).. Proenzim pepsin, pepsinogen, dilepaskan oleh sel utama pada dinding lambung, dan saat bercampur dengan asam klorida dari jus lambung, pepsinogen teraktifkan menjadi pepsin.
Estructura molecular de la enzima pepsina — Fotos de Stock © Raimund14 88817814
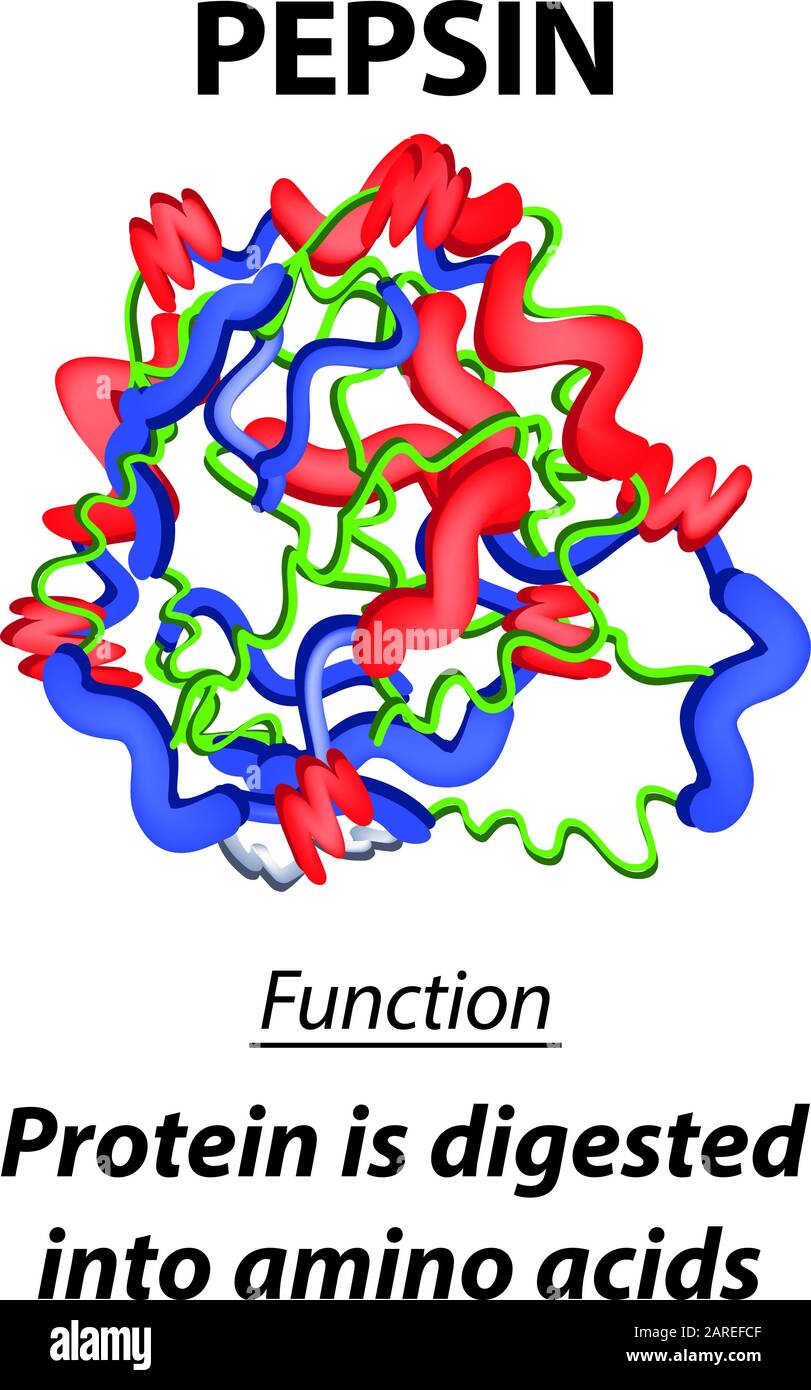
Asam hidroklorik inilah yang berfungsi mengubah pepsinogen, yaitu zat yang dibuat oleh sel-sel di lapisan dinding lambung, menjadi enzim pepsin. Fungsi Enzim Pepsin. Fungsi utama enzim pepsin adalah memecah struktur protein yang ada di dalam makanan menjadi asam amino. Hal ini berguna untuk mempermudah penyerapan nutrisi di dalam usus.
Apa Fungsi Enzim Pepsin?
Pepsin / ˈ p ɛ p s ɪ n / is an endopeptidase that breaks down proteins into smaller peptides and amino acids.It is one of the main digestive enzymes in the digestive systems of humans and many other animals, where it helps digest the proteins in food.Pepsin is an aspartic protease, using a catalytic aspartate in its active site.. It is one of three principal endopeptidases (enzymes cutting.
Gastric enzyme pepsin Stock Vector Images Alamy
Pepsinogen is a precursor of pepsin, a protease secreted in stomach, that the activation peptide assumes a compact structure that occludes the active site. Serum levels of pepsinogen and gastrin are parameters that can be used as biomarkers for gastric mucosa. On exposure to an acidic pH the activation peptide is cleaved, thereby unmasking the.
Pepsin enzyme function, source of production and where is pepsin found
Pepsin is a stomach enzyme that serves to digest proteins found in ingested food. Gastric chief cells secrete pepsin as an inactive zymogen called pepsinogen. Parietal cells within the stomach lining secrete hydrochloric acid that lowers the pH of the stomach. A low pH (1.5 to 2) activates pepsin.

Pepsinogen. Molecular model of pepsinogen, the inactive precursor to the digestive enzyme pepsin
Pepsin is one example of a group of enzymes termed "acid proteases." In the case of pepsin, this name is doubly appropriate. Pepsin works its best in strong hydrochloric acid. But the similarity with the other enzymes pictured here refers to a second type of acid. The active site of the acid proteases rely on two acidic aspartate amino acids.

Enzim Pepsin
Specific cells within the gastric lining, known as chief cells, release pepsin in an inactive form, or zymogen form, called pepsinogen. By doing so, the stomach prevents the auto-digestion of protective proteins in the lining of the digestive tract. Since chief cells release pepsin as a zymogen, activation by an acidic environment is necessary.
Enzyme pepsin 3D model stock illustration. Illustration of biochemistry 23665109
pepsinogen peptides is a proline at the N-terminal which was placed in the pepsinogen sequence to produce acid labile sequences and this proline is unlikely to affect any anti-microbial properties (16,17). Computational analysis was used to identify cryptic antimicrobial peptides (AMPs) based on amino acid composition, charge, and hydrophobicity of

Fungsi Enzim Pepsin Kumpulan Berita dan Informasi disekitar Kita
Pepsinogen. Pepsinogen is a powerful and abundant protein digestive enzyme secreted by the gastric chief cells as a proenzyme and then converted by gastric acid in the gastric lumen to the active enzyme pepsin. The role of pepsin and its precursor in protein digestion was first described in the 19th century.

Enzim Part 1 Biologi Ting 4 Bab 5 5.2 YouTube
pepsin, the powerful enzyme in gastric juice that digests proteins such as those in meat, eggs, seeds, or dairy products. Pepsin is the mature active form of the zymogen (inactive protein) pepsinogen.. Pepsin was first recognized in 1836 by the German physiologist Theodor Schwann.In 1929 its crystallization and protein nature were reported by American biochemist John Howard Northrop of the.
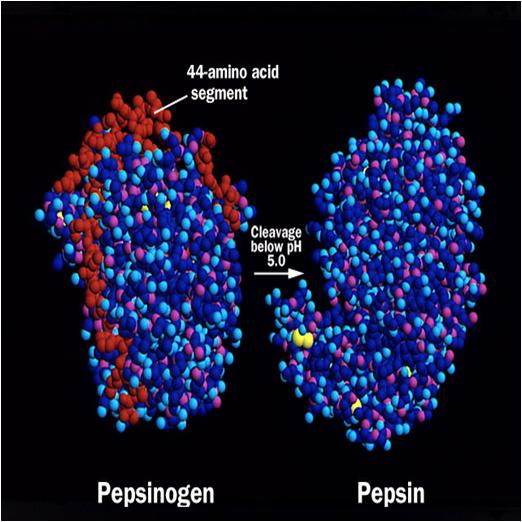
Difference between Pepsin and Pepsinogen
Purified pepsinogen converted into pepsin quickly at pH 2.0, and its optimum pH and temperature were 2, and 37 °C. Hence, ammonium sulfate with 67/5 % saturation showed the highest activity and.
Pepsin stomach enzyme Photograph by Science Photo Library Fine Art America
Pepsin. Jean-Pierre Raufman, in Encyclopedia of Gastroenterology, 2004. Structure and Activation of Pepsin. Like other aspartic proteinases (EC 3.4.23.X), pepsin (approximate molecular mass, 36 kDa) is synthesized as a proenzyme, pepsinogen (approximate molecular mass, 40 kDa), which is stable at neutral and alkaline pH (>6) and is converted to active pepsin at acid pH by proteolytic cleavage.